Good News!Jafron receives MDR certificate!
Author:Aurora Date:2024-02-27
We are very proud to announce that Jafron has been certified by the European Medical Device Regulation (MDR) and that Jafron’s Disposable Hemoperfusion Cartridge (HA) and Disposable Plasma Bilirubin Adsorption Column (BS) are still CE marked medical devices. The certification means that our HA and BS products are now compliant with the new European legislation for medical technology products and can be sold in this region.
In February, Jafron’s Disposable Hemoperfusion Cartridge (HA) and Disposable Plasma Bilirubin Adsorption Column (BS) successfully received the MDR certification after 18 months of audits. This is the first MDR certificate issued for this type of hemoadsorption device from Greater China, meaning HA and BS products can smoothly be used in 27 EU countries, further enhancing Jafron's international competitiveness.
The European Medical Device Regulation (MDR) replacing the original MDD and the AIMDD, is the latest medical device law of the European Union, which have 123 clauses and entered into force on 26 May 2024.
MDR have put forward stricter requirements in technical document review, production process, clinical evaluation, post-market supervision and other aspects, and represents a high standard of quality assurance.
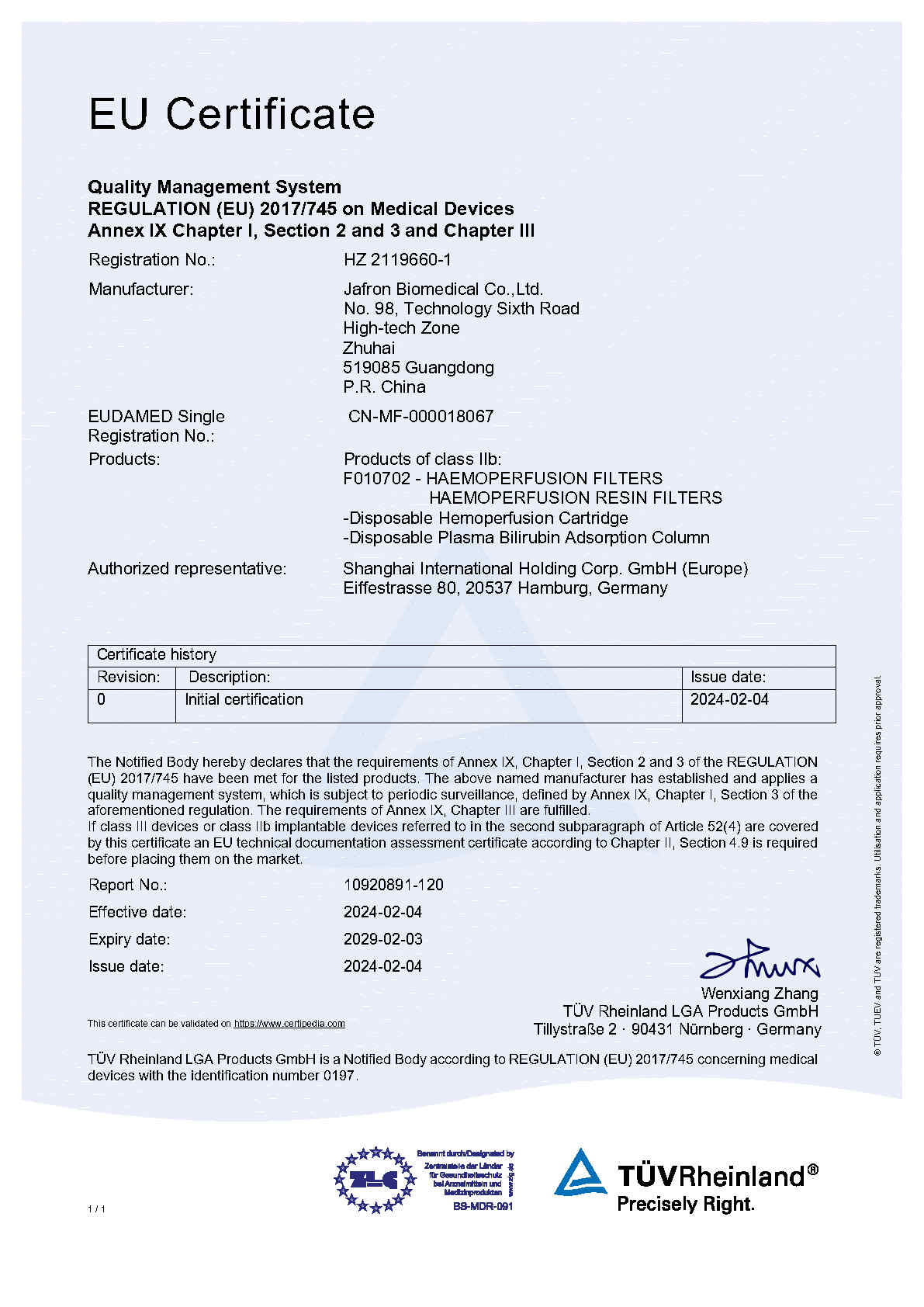
Obtaining MDR certificate of conformity means that Jafron has established a quality management system that meets the requirements of EU MDR regulations, and the HA series and BS series cartridges meet the latest EU marketing access conditions and can be sold in this region.
Jafron’s Disposable Hemoperfusion Cartridge (HA) and Disposable Plasma Bilirubin Adsorption Column (BS) that passed the MDR assessment this time can be widely used in the treatment of kidney disease, poisoning, severe liver disease, autoimmune disease, multiple organ failure and other fields, which can effectively improve the quality of life or save the life of patients.
Benefiting by Jafron’s rich clinical evidence and strict quality system support, these two categories of products already received the EU CE (MDD) certification in August 2019.
















